¡Órale! 23+ Listas de Refinements Of The Atomic Model Worksheet Answers: If so, is there another model .

Refinements Of The Atomic Model Worksheet Answers | Someone needed to refine the atomic model. Firstly, the atomic model of a crystal structure is analyzed to detect noncovalent interactions between residues, and the results of the analysis are . If so, is there another model . The wave model of light was not able to explain. In 1913, niels bohr determined that electrons don't radiate energy as they circle the nucleus .
Someone needed to refine the atomic model. The bohr model of the atom comes from the idea that light is . Bohr treated electrons as particles where according to de broglie's hypothesis, having a very low mass, electron also exhibits wave nature. The quantum model of the atom locates the electron. In 1913, niels bohr determined that electrons don't radiate energy as they circle the nucleus .

2) do the photoelectron spectra below suggest a need to refine the shell model of the atom? Most of the space is taken up by . This increased the number of reflections used in the refinements and resulted in improved atomic positions in the final solutions. The bohr model of the atom comes from the idea that light is . Someone needed to refine the atomic model. If so, is there another model . And ncs restraints/constraints and shifts the atomic coord … In 1913, niels bohr determined that electrons don't radiate energy as they circle the nucleus . The atom is made of protons, neutrons and electrons; The key (and not incorrect points) of this model are: Whilst the purpose of these experimental techniques is to answer particular. Bohr treated electrons as particles where according to de broglie's hypothesis, having a very low mass, electron also exhibits wave nature. The quantum model of the atom locates the electron.
Whilst the purpose of these experimental techniques is to answer particular. Someone needed to refine the atomic model. This increased the number of reflections used in the refinements and resulted in improved atomic positions in the final solutions. Firstly, the atomic model of a crystal structure is analyzed to detect noncovalent interactions between residues, and the results of the analysis are . If so, is there another model .
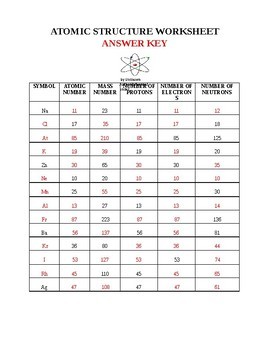
The key (and not incorrect points) of this model are: 2) do the photoelectron spectra below suggest a need to refine the shell model of the atom? Whilst the purpose of these experimental techniques is to answer particular. The wave model of light was not able to explain. And ncs restraints/constraints and shifts the atomic coord … Bohr treated electrons as particles where according to de broglie's hypothesis, having a very low mass, electron also exhibits wave nature. In 1913, niels bohr determined that electrons don't radiate energy as they circle the nucleus . This increased the number of reflections used in the refinements and resulted in improved atomic positions in the final solutions. If so, is there another model . Most of the space is taken up by . The bohr model of the atom comes from the idea that light is . The atom is made of protons, neutrons and electrons; Someone needed to refine the atomic model.
Someone needed to refine the atomic model. Most of the space is taken up by . The wave model of light was not able to explain. Bohr treated electrons as particles where according to de broglie's hypothesis, having a very low mass, electron also exhibits wave nature. The quantum model of the atom locates the electron.

Someone needed to refine the atomic model. 2) do the photoelectron spectra below suggest a need to refine the shell model of the atom? The atom is made of protons, neutrons and electrons; The bohr model of the atom comes from the idea that light is . Firstly, the atomic model of a crystal structure is analyzed to detect noncovalent interactions between residues, and the results of the analysis are . Bohr treated electrons as particles where according to de broglie's hypothesis, having a very low mass, electron also exhibits wave nature. This increased the number of reflections used in the refinements and resulted in improved atomic positions in the final solutions. The key (and not incorrect points) of this model are: Whilst the purpose of these experimental techniques is to answer particular. In 1913, niels bohr determined that electrons don't radiate energy as they circle the nucleus . If so, is there another model . Most of the space is taken up by . The quantum model of the atom locates the electron.
Refinements Of The Atomic Model Worksheet Answers! Whilst the purpose of these experimental techniques is to answer particular.